Trends
Medical Mission
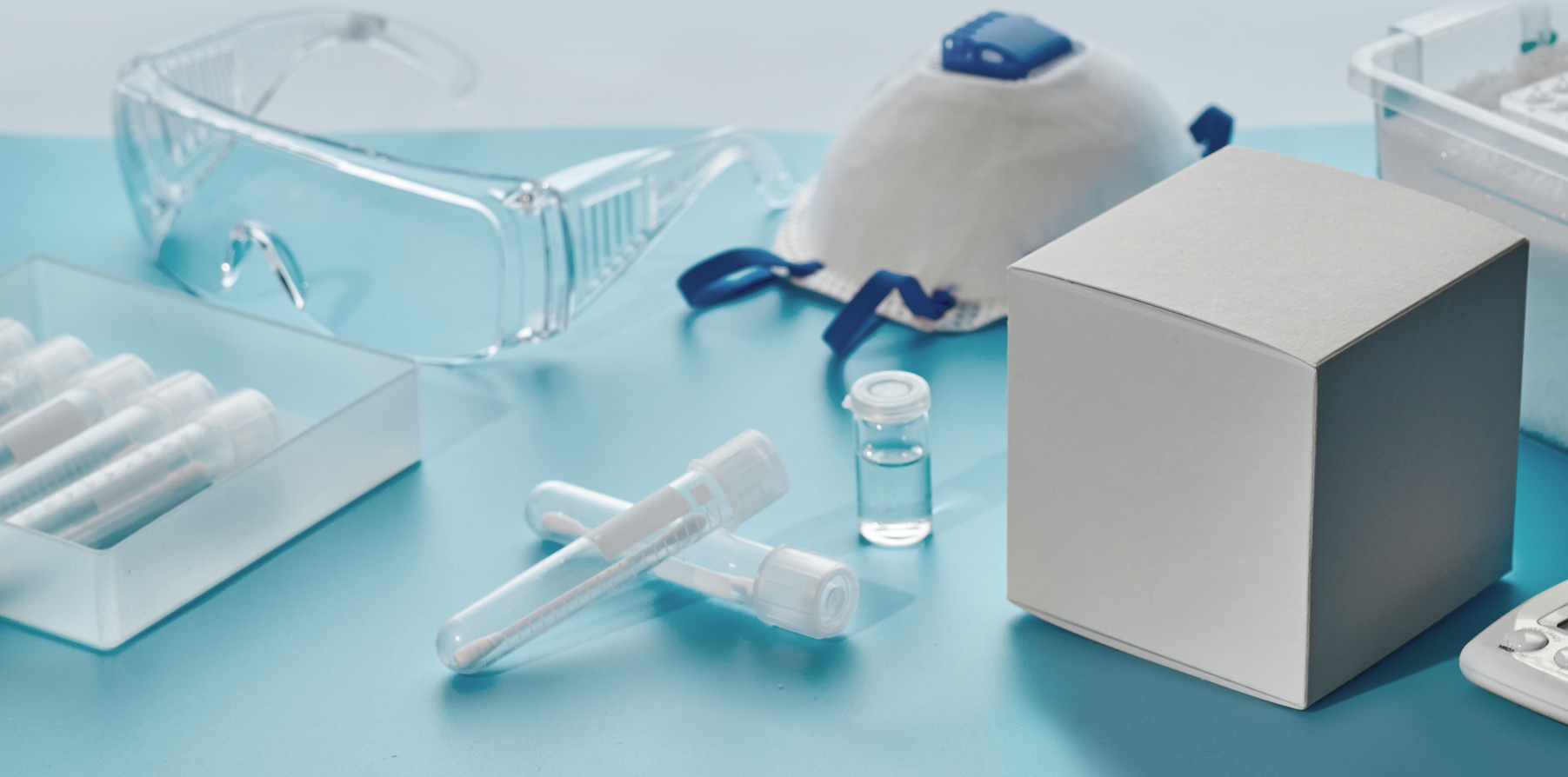
COVID-19 Test Kits
Despite the disruption of daily life in Korea and the world due to the COVID-19 pandemic, the crisis has provided a golden opportunity for Korean biomedical companies, which are developing test kits for the coronavirus, and the government, which has helped such businesses by issuing emergency approval and alleviating regulations. The world has since taken keen notice.
Written by• Lee Joon Ki,
Digital Times reporter
Global demand for diagnostic kits for COVID-19 made in Korea has skyrocketed since the start of the outbreak. The kits display the ingenuity and proficiency of domestic bio companies and the government thanks to the former’s prompt R&D and the latter’s strategic administrative handling of medical approval.
As of April 24, 53 test kits, the most from any nation, had been approved for export by the Ministry of Food and Drug Safety. Among the kits, those made by five companies were authorized for emergency use by the government, and this ignited high interest in the kits from abroad. To meet the surging demand for their cutting-edge products, the companies operated production at a nonstop pace.
The five Korean manufacturers of test kits — Seegene, SD Biosensor, Osang Healthcare, Seasun Biomaterials and LabGenomics — also had their kits authorized for emergency use by the U.S. Food and Drug Administration. Another kit maker, SolGent, is registered with the U.S. Federal Emergency Management Agency as a supplier for stockpile procurement. The U.S. is among the 40-plus countries where the six companies export their products.
According to the Korea Customs Service, exports of Korean test kits in April reached USD 212.3 million, up nearly 800% from USD 24.1 million in March. The number of markets also leaped from just one in January to 33 in February, 81 in March and 103 in April.
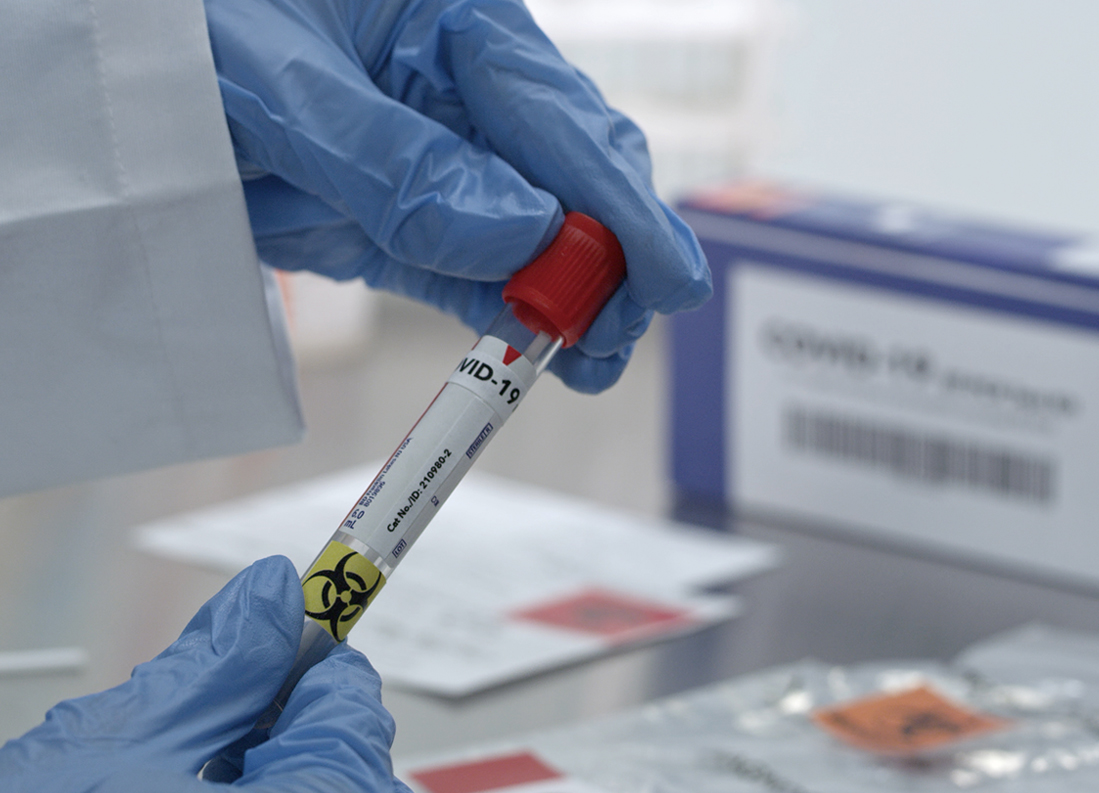
The Ministry of Food and Drug Safety has quickly approved test kits for emergency use after conducting thorough clinical evaluations. © shutterstock
Meeting Global Standards
The COVID-19-related protocols of the Korean government have been widely shared, with select ones being refined to meet global standards.
The country’s comprehensive method of handling suspected and confirmed cases of the pandemic — diagnostics (those who test positive) → contact tracing → quarantine (treatment) — will be submitted to the International Organization for Standardization for global standardization. Innovative methods like drive-thru and walk-thru screening clinics and community treatment centers for patients without severe symptoms are included in the submission.
World-class experts are scrutinizing and assessing the standardization process. In February this year, a COVID-19 screening methodology (real-time RT-PCR) was approved as a draft international standard and final approval is slated for November.
Pushing Proactive Administration

Korea has implemented diagnostic procedures for COVID-19 with speed and efficiency. © YonhapNews / The development of COVID-19 diagnostic kits has progressed at breathtaking speed, with such kits being constantly enhanced to ensure prompt and accurate testing.© YonhapNews
Experts credit the Korean government for enabling many aspects of the country’s defense against COVID-19. Astute and responsive administration led to strategic responses that earned international acclaim.
Authorization for emergency use against COVID-19 allowed the approval of relevant resources in just one week, far faster than the conventional period of 80 days or more. As a result, up to 15,000 people were tested daily for the coronavirus. Not only did this lead to increased exports, but the spread of COVID-19 nationwide was curbed.
In addition, the government set up a system for the orderly purchase of protective masks, community treatment centers for patients with mild symptoms and call centers for health counseling, and the nimble application of hospital mask filters. Minister of Trade, Industry and Energy Sung Yun-mo said, “The international benchmarking of Korea’s administrative handling of the crisis will not only elevate our national status, but also serve as a catalyst for our bio-health industry to claim superiority on the world stage.”
Fostering Major Players
Korea is a step ahead in just about every method for combating COVID-19, even preventive measures. Its bio-health sector is conducting R&D for vaccination and medication. For example, the iopharmaceutical company Celltrion is developing a treatment for COVID-19. Once its cell line is developed, the company will start from July clinical trials in Europe. If no issues or hazards are found, Celltrion is set to emerge as a global leader in R&D for COVID-19 antibodies.
Bukwang Pharmaceutical, with extensive experience in drug development, has received government approval for second-phase trials of Levovir, a drug for treating hepatitis B. Results of the trials will determine when R&D for the promising drug begins.
The clinical trials not only target treatment of COVID-19 but also vaccination. A consortium of medical companies and think tanks, several of which are government-run, are conducting nonclinical trials for the DNA vaccine drug GX-19. The consortium comprises Genexine, Binex, the International Vaccine Institute, GenNBio, the Korea Advanced Institute of Science and Technology, and Pohang University of Science and Technology. Genexine’s recent trials of GX-19 on primates confirmed the drug’s ability to generate neutralizing antibodies. The consortium plans to register for conducting human clinical trials to pioneer vaccines for the coronvirus based on GX-19 substances.
National resources are also going into R&D for COVID-19 vaccines and medication. Seven clinical trials for medication — specifically drug regeneration — are in progress and set for completion around year’s end. In vaccination, three drugs will undergo clinical trials before next year, with the aim to produce vaccines in the latter half of 2021.

Researchers at Institut Pasteur Korea in Seongnam, Gyeonggi-do Province, work on chemical compounds to fight COVID-19. Korean medical companies and institutes alike are racing to develop vaccines against the coronavirus. © YonhapNews